HISTORICAL HIGHLIGHT
Innovation and Invention
In recent years, “technology transfer” has become a major goal of academic institutions. The activities that compromise technology transfer, such as filing of patents, enforcement of licensing rights, and fostering of start-up companies, were traditionally activities based in the commercial sphere. However, this paradigm began to shift with the advent of the Bayh-Dole Act of 1980, which legislated that recipients of federal funding, for example universities, could patent and license inventions resulting from federally funded research. This law was intended to encourage the dissemination of academic research for commercial use. As a result, patenting and licensing of new technologies emerged as a tool used by universities to invest in the R&D of clinically applicable discoveries. Columbia was one of the first universities to take up the government’s challenge and implement a program to encourage commercialization of research, forming the office now known as Columbia Technology Ventures (CTV) in 1982. Moreover, researchers in the Department of Microbiology & Immunology have proven to be pioneers in the technology transfer sphere at Columbia University, claiming two of the most successful sets of patents in Columbia’s history.
Inspiration from Past Innovators to the Innovators of Tomorrow
CTV has received 129 inventions from the Department of Microbiology & Immunology since 1983, when they began to track departmental contributions, and Microbiology & Immunology patents have generated over 30 license agreements. Two patent families in particular, the Co-transformation patents and the Chimeric Monoclonal Antibody patents, have been widely licensed and are behind many of the largest biotechnology therapies of the past two decades.
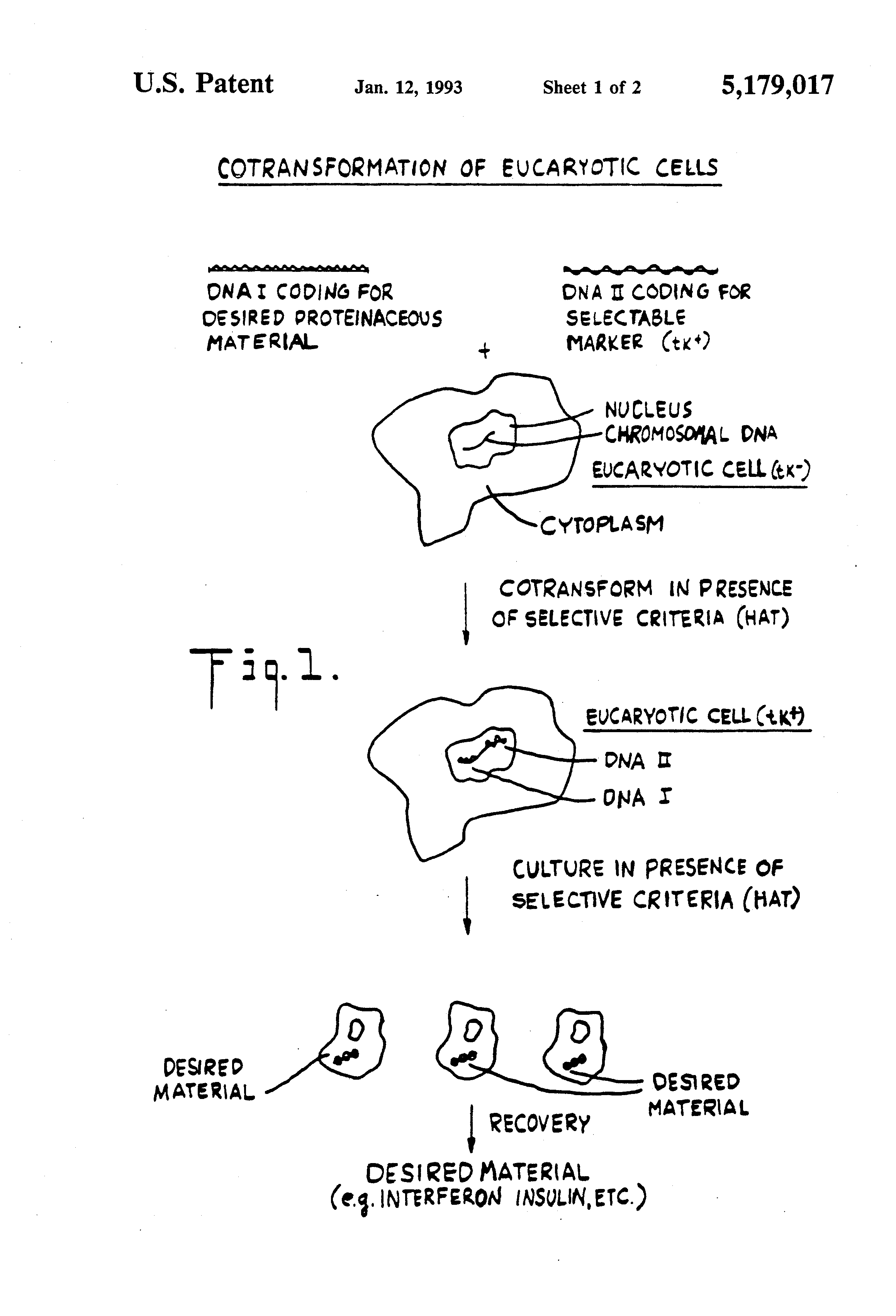
The Co-transformation patents are among the most lucrative and widely recognized biomedical patents in Columbia history. Even before the passage of the Bayh-Dole Act, at a time when patenting of biomedical technologies was uncommon, three scientists from Columbia University – Dr. Michael Wigler, Dr. Saul Silverstein, and Dr. Richard Axel – not only devised a novel technique, but had the forethought to patent their discovery. The patents were granted for the method of co-transformation. As a student in the Silverstein lab, Wigler, with the guidance of his mentor Dr. Silverstein, first introduced the thymidine kinase (tk) gene from the Herpes Simplex Virus genome into a mammalian cell line. This process may seem commonplace today, but Wigler and Silverstein were among the first to demonstrate expression of a functional protein by gene transfection in a eukaryotic cell system. Since cells lacking tk are unable to grow in HAT media, Wigler further demonstrated that culturing of the transfected cells in HAT media led to selection of cells that stably-expressed the tk gene over several hundred cell generations. His initial paper was published in Cell in 1977. Dr. Axel then contributed to the subsequent work by suggesting that co-transformation of a gene of interest along with a selectable gene, such as the HSV tk gene, could lead to stable expression of the gene of interest, for which there was no selection pressure. The resulting paper describing this revolutionary process of co-transformation was published in Cell in 1979. It was for this innovation, that Wigler, Silverstein, Axel, and a fourth innovator, Dr. James Roberts, submitted their first patent. Ultimately, Columbia University was granted five major patents for the co-transformation technique and related applications in 1983, 1987, 1993, and 2002.
Since the initial invention, co-transformation has become a widely used method that has contributed to the development of numerous commercial technologies. Silverstein recalls, somewhat ironically, that, “We actually thought it might be valuable, but we weren’t sure.” Genentech was the first company to license the technology, and then other biotech and pharmaceutical companies followed suit. Many products stemmed from the technology, but the most successful by far was Epogen, developed by Amgen. Epogen is a recombinantly produced human erythropoietin that is used to treat anemia associated with chronic renal failure and chemotherapy. Among the many other important therapies that stemmed from the technology were two products developed by Genentech: Activase, a recombinant tissue plasminogen activator (tPa), is widely used to dissolve blood clots in heart attack patients, and Pulmozyme, a purified solution of human deoxyribonuclease I (rhDNase), is used to reduce viscosity in the lungs of cystic fibrosis patients.
The other major set of patents that stem from the Department of Microbiology & Immunology are for the development of chimeric antibodies, developed, in part, by a former faculty member, Sherie Morrison. For work done both at Columbia and while on sabbatical at Stanford University, Morrison is credited with being a co-inventor of a system to produce antibodies in cells and for developing a technique to create antibodies with both mouse and human components. In 1982, Morrison’s lab developed a method to produce antibodies in lymphoid cells. They were able to identify lymphoid specific regulatory elements that controlled antibody expression and used this knowledge to create expression vectors to produce antibodies in cell culture. The primary goal was to make antibody fusion proteins with specific functional properties.
Monoclonal antibodies were used in the early 1980s for various applications, but were often produced in rodents, and were therefore immunogenic to other species. It was known that the constant region of antibodies had functions in complement binding, immunogenicity, and cell receptor binding. Thus, it was thought that a chimeric antibody containing a human constant region and a variable binding region specific for a particular ligand, as developed in a mouse cell system, could have clinical applications. These chimeric antibodies turned out to be better tolerated by the human immune system than rodent antibodies. Morrison and her colleagues attempted to patent the chimeric antibody technology in 1984, but the two patents were not issued until 1998 and 2001. This turned out to be advantageous since the patents ultimately went into effect at a time when the biotech and pharmaceutical industries were eager to adopt the technology.
The chimeric antibody technology has since been widely licensed and used by pharmaceutical companies to create various clinical products. For example, Centocor (now part of Johnson & Johnson) used the method to replace parts of a mouse antibody with human domains that recognize a cell adhesion molecule. The resulting drug is known as ReoPro and has been approved by the FDA to reduce clots and subsequent heart problems during heart surgeries. The Morrison patents have also lead to well-known products such as Remicade, a chimeric antibody against TNFα. Remicade was developed by researchers at Centocor and New York University (NYU) and is used for the treatment of Crohn’s disease and rheumatoid arthritis.
In addition to the vast clinical applications of the Columbia-created technologies, an invaluable consequence of the patenting and licensing of the co-transformation and chimeric antibody production methods was the collection of licensing fees and royalties by the University. As with all patent revenue at Columbia, the significant revenue from these two sets of patents were divided to reward the inventors, to fund further research in the laboratories’ of the inventors, and to be used for both the upholding of the patents and for general university expenses. It has been reported by Jeffrey Kestler, associate general counsel of the Patent and Licensing Group at Columbia University, that the University’s share of the licensing revenue from the co-transformation patent contributed to the establishment of various new programs, including the Department of Biomedical Engineering at the Fu School of Engineering, the J.P. Sulzberger Columbia Genome Center, and the Columbia University Earth Institute, among others.
The co-transformation and chimeric antibody patents also generated revenue for the University, the medical school and the Department of Microbiology & Immun-ology, which has supported decades of research and development. The patent money was used to establish four endowed professorships in the department and to set up a student fund for purposes such as purchasing laptop computers for incoming students. Funds were used to purchase computers for various labs, a depart-mental server, and departmental research software, such as Openlab and Volocity. Finally, the revenue was also used to fund the annual department retreat, which fosters collegiality and collaboration between the faculty, postdoctoral fellows, and students.
Despite our department’s history of commercial success in translating laboratory discoveries to patents, it is important to remember that not all patenting and licensing attempts end with blockbuster victories. Patentability of a discovery is determined by how a technology is developed, when it is made public, and how it is protected during discussions with companies, that can sometimes cause even the best ideas to go unpatented or unlicensed. Commercializing intellectual property poses challenges, as well. According to the Association of University Technology Managers (AUTM), only 18% of invention disclosures turn into an active license, option, or startup. Of those licensed, less than one percent end up generating more than $1 million in licensing revenues annually. Even at Columbia, where gross tech transfer revenues have consistently been in the top five among U.S. universities, patents often take a long time to get licensed, and many don’t get licensed at all. For example, the late Dr. Bernard Erlanger, a professor in our department, was a prolific inventor who was successful in obtaining over a dozen patents for innovations such as methods for screening anti-HIV compounds and novel treatment protocols for sickle cell disease. Of Dr. Erlanger’s many inventions, one was licensed non-exclusively multiple times, and a second invention was recently licensed to a startup company, unfortunately only after Dr. Erlanger’s passing.
Experience has proven that there is an element of luck in achieving commercial success. To maximize the chance of realizing the commercial potential of our work, Dr. Ofra Weinberger, Director at CTV, encourages the inventors among us to submit invention reports early and often and reach out to your licensing officers regularly. Regardless of commercial outcome, however, the utility of working hard to create something of importance, be it a new technology or a better understanding of how a biological process works, should never be undervalued. As scientists, we proceed with the certainty that our work has significance and the belief that investment in our work will one day pay off, in one way or another.